Actinobacillus succinogenes strain 130Z (ATCC 55618) was isolated from the bovine rumen by MBI Interactional. It is a Gram-negative, facultatively anaerobic, pleomorphic bacterium. A. succinogenes belongs to the family Pasteurellaceae that, in addition to Actinobacillus, includes the genus Mannheimia, Haemophilus, and Pasteurella. These bacteria are generally pathogenic or commensal. A. succinogenes is thought to serve a commensal role producing organic acids that are used as an energy source by the cow (1) . One of the major endproducts of its fermentative metabolism is succinate. Succinate has many industrial fine chemical uses (4). As a specialty chemical, it is a flavor and formulating ingredient in food processing, a pharmaceutical ingredient, and a surfactant. Succinate's greatest market potential, though, would be its use as an intermediary commodity chemical feedstock for producing bulk chemicals, stronger-than-steel plastics, ethylene diamine disuccinate (a biodegradable chelator), and diethyl succinate (a green solvent for replacement of methylene chloride) (4). These bulk chemicals, plastics, solvents, as well as succinate are currently produced petrochemically from butane through maleic anhydride. More than 17,000 tons of succinate are sold per year. It is currently sold in the U.S. for $2.70-4.00/lb, depending on its purity. It can also be made by fermentation from glucose at a production cost of about $1.00/lb. For succinate to be competitive with maleic anhydride as a commodity chemical, its overall production cost should be lowered to approximately 15 cts/lb. World-wide sales of biobased products have increased from $8 billion in 1983 to almost $14 billion in 1994. Current prospects for biobased products are for a $100 billion market, with a prospect for more than $15 billion in products from succinate. Only 20% of the U.S. corn crop is needed to replace all chemicals made from petroleum in the U.S. Decrease in U.S. corn exports in the past decade have been balanced by new industrial uses, and U.S. la nd capacity exists to produce >40% more corn. For these reasons, using biobased succinate as an intermediary chemical for the production of biobased products represents a highly attractive alternative to synthetic processes based on fossil fuels of foreign origin. Because A. succinogenes is the best known natural succinate producer, because it utilizes a wide range of substrates (including glucose, cellobiose, lactose, xylose, arabinose, and fructose), and because it has a high tolerance for elevated concentrations of succinate salts, it is well suited for industrial succinate fermentation. For every mole of succinate made by A. succinogenes , a mole of CO2 is fixed. Increased CO2 availability has been shown to increase succinate production by A. succinogenes (3) . Some of the potential industrial products of succinate include plastics, meaning that the CO2 fixed is not immediately returned to the atmosphere. Some have envisioned coupling an industrial succinate fermentation to an industrial ethanol fermentation to capture CO2 waste from the ethanol fermentation and trap it in useful chemical product (4). Succinate production can be increased in A. succinogenes by diverting carbon flux away from alternative endproducts such as acetate and formate. The genome sequence will undoubtedly be valuable for modeling metabolic pathways and designing informative experiments to study metabolic fluxes and metabolic controls. The outcomes of these studies will be used to direct the engineering (2) of highly productive succinate producing strains of A. succinogenes for industrial succinate production. References1. Guettler, M. V., D. Rumler, and M.K. Jain. 1999. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int. J. Syst. Bacteriol. 49:207-216. 2. Kim, P., M. Laivenieks, J. McKinlay, C. Vieille, and J.G. Zeikus. 2004. Construction of a shuttle vector for the overexpression of recombinant proteins in Actinobacillus succinogenes . Plasmid 51:108-115. 3. van der Werf, M. J., M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1997. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp . 130Z. Arch. Microbiol. 167:332-342. 4. Zeikus, J. G., M.K. Jain, P. Elankovan. 1999. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Environ. Microbiol. 51:545-552. Other References 1. Kim, P., M. Laivenieks, C. Vieille, and J.G. Zeikus. 2004. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli . Appl. Environ. Microbiol. 70:1238-1241. 2. Guettler, M. V., M.K. Jain, and B.K. Soni. 1996. Process for making succinic acid, microorgansims for use in the process and methods of obtaining the microorganisms. U.S. patent 5,504,004. 3. Guettler, M. V., M.K. Jain, and D. Rumler. 1996. Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. U.S. patent 5,573,931. 4. McKinlay, J. B., J. G. Zeikus, and C. Vieille. 2005. Insights into Actinobacillus succinogenes fermentative metabolism in a chemically defined growth medium. Appl. Environ. Microbiol. 71:6651-6656. 5. McKinlay, J.B., Shachar-Hill, Y., Zeikus, J.G., and C. Vieille. 2007. Determining Actinobacillus succinogenes metabolic pathways and fluxes by NMR and GC-MS analyses of 13 C-labeled metabolic product isotopomers. Metab. Engin. doi:10.1016/j.ymben.2006.10.006. 6. Park, D. H., M. Laivenieks, M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1999. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl. Environ. Microbiol. 65:2912-2917. 7. Park, D. H., and J. G. Zeikus. 1999. Utilization of electrically reduced neutral red by Actinobacillus succinogenes : physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 181:2403-2410. |
||
|
||
Actinobacillus succinogenes 130Z

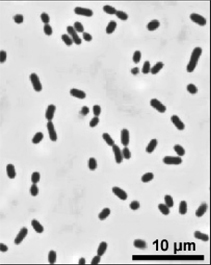 Photo: James McKinlay, Michigan State University
Photo: James McKinlay, Michigan State University