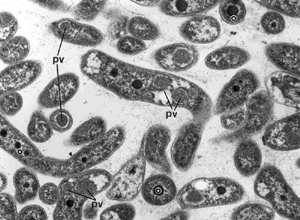
The root and stem-nodulating bacteria of the genera Bradyrhizobium, Allorhizobium, Sinorhizobium, Mesorhizobium, and Rhizobium are prime targets for genome analysis due to their relatively large genomes, environmental elasticity, diverse growth modes, and their ability to establish nitrogen-fixing symbioses which enhance plant growth [1]. Several Bradyrhizobium - related species have the unusual capacity to form nodules on stems of aquatic legumes from the Aeschynomene genus [2]. This monophyletic rhizobial clade is of special interest because of its unique capacity for photosynthesis [3-5]. They synthesize photosynthetic pigments including both bacteriochlorophyll a and carotenoids, and contain photosynthetic reaction centers like those of the purple nonsulfur photosynthetic bacteria [4,5]. Photosynthetic activity plays a key role in symbiosis by furnishing energy, which can be used for biological nitrogen fixation [4,5]. The regulatory mechanisms for the formation of this photosystem are very different from those described in purple bacteria, and include a bacteriophytochrome, which, as a function of the ambient light, triggers or fails to trigger the expression of photosynthetic genes [5]. This original mechanism of regulation seems especially well adapted for promoting specific photosynthetic activity during symbiosis with the stem [5]. References:
|
||
|
||
Bradyrhizobium sp. BTAi1