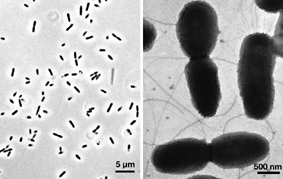
Verminephrobacter eiseniae EF01-2 is associated with earthworm nephridia in a poorly understood animal/bacterium symbiotic system. V. eiseniae EF01-2 belongs to a group of recently isolated bacteria, inhabitants of the earthworm nephridia (excretory organs present in every segment of the worm). Recognized cases of annelid-bacteria symbioses are numerous, diverse and ecologically important (reviewed in Bright and Giere, 2005). These include, among others, the endosymbiotic communities of gutless aquatic oligochaetes from the genus Olavius (Dubilier et al., 1995); the obligate association between the Vestimentiferan worm Riftia pachyptila and a sulfur-oxidizing chemoautotrophic symbiont (Cavannaugh et al., 1981); and the esophageal gland endosymbionts of leeches (Kikushi and Fukatsu, 2002). With the exception of those conferring sulfur-based chemolithotrophic capacity on their hosts, little is known about the physiological basis for these associations. One of the limitations for the study of these associations is the inability to experimentally manipulate the symbiont and host in the laboratory (Bright and Giere, 2005). Thus, the earthworm-"Verminephrobacter" association is a rare example of a tractable system among known annelid symbioses, and one of the few animal-bacteria symbioses recognized to involve a betaproteobacterium (Thao et al., 2002; Horn et al., 2002). Earthworms represent a highly successful group of terrestrial annelids (5000+ described species) in the class Clitellata that occur naturally on all continents except Antarctica and a few insular archipelagoes. Earthworms are ecosystem "engineers", playing important roles in the processing of soil. Their activities alter soil structure, nutrient availability, and microbial activities (Edwards and Bohlen, 1996; Edwards, 2004). The relatively transient microbial population in the gut, apparently originating from the soil, contributes to transformation of organic material and the production of nitrous oxide, a known greenhouse gas (Karsten and Drake, 1997; Matthies et al., 1999). Although the transient populations of the gut may influence host digestive function, we anticipate that the more intimate bacterial symbionts of the nephridia have significant adaptive significance. The availability of the genome sequence for the bacterial symbionts present within the earthworm nephridia will facilitate a deeper understanding of the biology of the association, and by extension a greater comprehension of the biology of these important members of soil ecosystems. |
||
|
||
Verminephrobacter eiseniae EF01-2