Note: Name change from
Chlorochromatium aggregatum to Chlorobium chlorochromatii CaD3 (10/19/05).
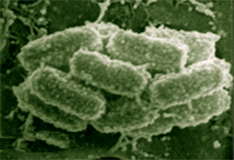
Phototrophic consortia, structural associations between a colorless central bacterium and surrounding green- or brown-pigmented epibionts, were first described about 100 years ago. To date, seven different morphotypes of motile, phototrophic consortia have been repeatedly observed in freshwater lakes around the world (1, 2). Such consortia can reach high concentrations in some environments, and it is believed that they represent important components of the carbon and sulfur cycles in freshwater environments. Recently, Fröstl and Overmann (3) established the first stable enrichment culture of a phototrophic consortium, “Chlorochromatium aggregatum.” Electron microscopy had demonstrated the presence of chlorosomes in the epibionts of “C. aggregatum,” providing strong presumptive evidence that the epibionts were green sulfur bacteria. Using highly specific oligonucleotide hybridization probes and PCR primers for 16S rRNA, the phylogenetic affiliation of the epibionts with the green sulfur bacteria was verified (4). Studies of the environments in which phototrophic consortia are found suggest that the epibionts are not derived from the free-living green sulfur bacteria also present but instead represent novel phylotypes. The metabolic interactions between the partners in phototrophic consortia are still unknown. All green sulfur bacteria studied to date are obligate anoxygenic photolithotrophs which can typically utilize hydrogen, sulfide, elemental sulfur or polysulfide, and sometimes thiosulfate as electron donors for carbon dioxide fixation by the reverse TCA cycle. Most strains show a very limited ability to photoassimilate simple organic compounds (acetate, pyruvate, propionate, lactate, succinate, etc.). Interestingly, “C. aggregatum” is chemotactic towards 2-oxoglutarate, and this compound was found to be an essential ingredient of the enrichment medium for this consortium (3). Green sulfur bacteria and sulfate- or sulfur-reducing bacteria can be maintained in highly stable cocultures because of the establishment of a closed sulfur cycle in which each sulfur atom cycles many times. Because sulfide concentrations are quite low in the natural environments in which phototrophic consortia typically are found, it has been suggested that a syntrophic sulfur cycle may also exist between the two partner organisms in these consortia. Such an arrangement would probably insure a continuous supply of reduced sulfur compounds for energy and reducing power generation by the epibionts. It is possible that the epibionts may provide reduced carbon or nitrogen to the motile central rod. Sulfide has been shown to stimulate electron transport in the epibionts. However, present evidence indicates that the central rods of the consortia may not be a classical sulfur- or sulfate-reducing bacterium. These metabolic capabilities are largely but not exclusively confined to the d-subgroup of the proteobacteria, and recent molecular analyses conclusively demonstrate that the central rod of phototrophic consortia are members of the b-subgroup of the proteobacteria (5). A number of other observations indicate very close and controlled interactions between the phototrophic epibionts and the central rod-shaped partner. For example, the number epibionts per consortium, which in the case of “C. aggregatum” is 12.9 ± 4.5 epibionts per central rod, exhibits a non-random frequency distribution with a distinct maximum. This strongly implies that cell division of the epibionts occurs in a coordinated fashion. Moreover, microscopic studies indicate that the central chemotrophic bacterium grows and divides while in association with the epibionts. Taken together, these observations strongly imply that cell division of the two partners proceeds synchronously. However, nothing is known of the cell-to-cell signals that could coordinate such events. The most striking evidence of cell-to-cell communication between the two partners is the observation that consortia exhibit a scotophobic response and can accumulate on the basis of this phototactic response at a wavelength of 740 nm (3). Since this wavelength corresponds to the absorption maximum of bacteriochlorophyll c, the major photosynthetic pigment of the epibiont, the photosynthetic pigments appear to be the photoreceptors for the scotophobic response. Since only the central rod is flagellated and motile, these observations therefore imply that a rapid, interspecies cell-to-cell communication mechanism must occur between the nonmotile, green colored epibiont and the motile, colorless central rod-shaped bacterium. Understanding the molecular bases for the syntrophic metabolic interactions, for the coordinated cell division, and for the interspecies cell-to-cell signaling of phototaxis are major objectives of determining the genome sequences of the two partners. “C. aggregatum” can be cultured in the laboratory, but has not yet been rendered axenic. However, because of its size, the consortium can be separated from the one other contaminating bacteria found in the enrichment cultures. Moreover, the components of the consortium can be disrupted and separated to produce enriched fractions representing the green-sulfur epibiont and the colorless central rod. An exciting recent development has been the ability to cultivate the epibiont free from the central rod (J. Overmann, unpublished results). This allows DNA from the epibiont to be easily purified. Available genome mapping data suggest that the genomes of green sulfur bacteria span a narrow range between 2 and 3.3 Mb (Mendez-Alvarez et al., 1995). A survey of the TIGR web site indicates that the size range for genomes of b-proteobacteria is 2 to 6 Mb. Thus, the it is anticipated that the total genome size for “C. aggregatum” is less than 10 Mb. Because this consortium has a well-defined number of epibionts per central rod (~13:1), this system provides an interesting test case for the feasibility of sequencing environmental samples in which the ratio of cell types is not known. References:
|
||
|
||
Chlorobium chlorochromatii CaD3