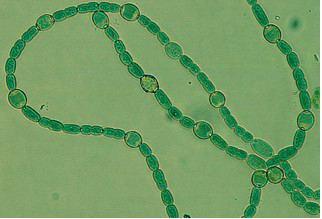
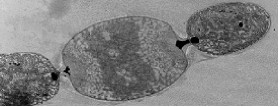
This organism is now known as 'Nostoc azollae' 0708, (9/12/08). The Azolla cyanobiont produce combined nitrogen, and as a side-reaction, the nitrogen-fixing enzyme also produces hydrogen gas, a potentially important bio-gas (Lechno-Yossef & Nierzwicki-Bauer 2002; van Hove & Lejune 2002). These features, combined with a fast growth using solar energy only, make biomass production and energy conversions (e.g. into bio-fuels) via the Azolla symbiosis energetically attractive. The cyanobiont of Azolla is taxonomically well rooted close to the nitrogen-fixing genera Nostoc and Anabaena (Svenning et al. 2005, Ekman et al. 2007). The cyanobiont of Azolla has a flexible life style including symbiotic competence and ability to differentiate several cell types from the photosynthetic vegetative cells: hormogonia (short, small-celled and motile filaments), nitrogen-fixing heterocysts and resting akinetes (spores), all cell-types seen in the Azolla symbiosis (Zheng et al. 2008). The Azolla host is a heterosporous fern (genome size of about 720 Mb) and represents a wide-spread and fast-growing plant with a doubling time of 2-5 days (van Hove & Lejune 2002).Seven species are recognized. The cyanobacteria colonize ‘cavities’ found in all dorsal leaves of the Azolla plant (Lechno-Yossef & Nierzwicki-Bauer 2002). The plant supplies the cyanobiont with fixed carbon, and receives a perpetual source of combined ‘new’ nitrogen from the nitrogen-fixing cyanobiont (Meeks et al. 1988). The Azolla cyanobiont also shows unique characters. For instance, the cyanobiont is vertically transmitted into new plant generations within the plant reproductive organs, the sporocarps (Perkins & Peters 1993; Zheng et al. 2008). Also, the cyanobiont seems to have lost its capacity for independent growth (Lechno-Yossef & Nierzwicki-Bauer 2002). We therefore hypothesize that Azolla is evolutionarily the most advanced extant plant symbiosis and potentially on its way to develop into a plant with a nitrogen-fixing ‘organelle’. The Azolla symbiosis has been used to sustain agricultural productivity in south-east Asia for over a thousand years (van Hove & Lejune 2002). The symbiosis is involved in vital processes such as energy capture and production of energy rich carbohydrates via photosynthesis (carbon sequestration) and combined nitrogen fixation (nitrogen sequestration), processes fed by solar energy. The Azolla symbiosis thereby represents a most self-renewable source of energy and key nutrients. An understanding of the cyanobacterial genome may open new perspectives for biotechnological innovation related to the production of N-fertilizers and bio-energy conversions and may help alleviate our dependence on industrially and oil-based nitrogen. It will also provide information in exciting areas of biology, notably in evolution and inter-kingdom interactions.
References
|
'Nostoc azollae' 0708